V.
Drugs That Effect
Cholinergic Mechanisms (Part 2).
Outline
D. Anticholinesterase Agents
1. Neostigmine and Pydrostigmine
2. Serine Nerve Gas
E. Acetylcholine Antagonists
1. Atropine
2. Curare
3. Botulus Toxin
D.
Anticholinesterase Agents.
Acetylcholine is inactivated by the enzyme acetylcholinesterase
(enlarged),
which is located at cholinergic synapses and breaks down the acetylcholine molecule into
choline and acetate. Three particularly well-known drugs, neostigmine,
physostigmine, and diisopropyl fluorophosphate, inactivate acetylcholinesterase so
that it cannot hydrolyze the acetylcholine released at the nerve ending. As a result,
acetylcholine increases in quantity with successive nerve impulses so that large amounts
of acetylcholine can accumulate and repetitively stimulate receptors. In view of the
widespread distribution of cholinergic neurons, it is not surprising that the
anticholinesterase agents as a group have received more extensive application as toxic
agents, in the form of agricultural insecticides and potential chemical-warfare
"nerve gases," than as therapeutic agents. Nevertheless, several members
of this class of compounds are clinically useful.
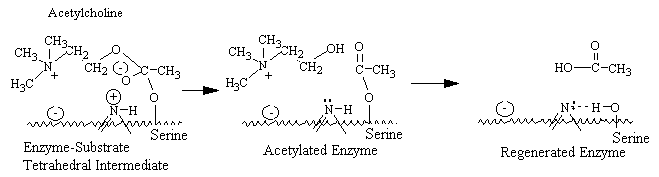
The active center of acetylcholinesterase consists of a
negative subsite, which attracts the quaternary group of choline through both coulombic
and hydrophobic forces, and an esteratic subsite, where nucleophilic attack occurs on the
acyl carbon of the substrate. The catalytic mechanism resembles that of other serine
esterases, where a serine hydroxyl group is rendered highly nucleophilic through a
charge-relay system involving the close apposition of an imidazole group and , presumably,
a carboxyl group on the enzyme. During enzymatic attack on the ester, a tetrahedral
intermediate between enzyme and ester is formed that collapses to an acetyl enzyme
conjugate with the concomitant release of choline. The acetyl enzyme is labile to
hydrolysis, which results in the formation of acetate and active enzyme.
Acetylcholinesterase is one of the most efficient enzymes known and has the capacity to
hydrolyze 3 X 105 acetylcholine molecules per molecule of enzyme per minute:
this is equivalent to a turnover time of 150 microseconds.
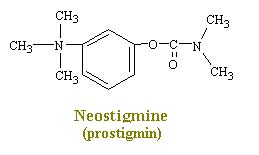
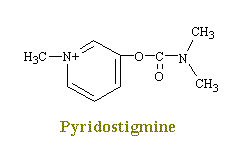
Drugs such as neostigmine and pydrostigmine that have a
carbamyl ester linkage are hydrolyzed by acetylcholinesterase, but much more slowly than
acetylcholine because they from more stable intermediates. Therefore, these drugs
inactivate acetylcholinesterase for up to several hours, after which they are displaced so
that acetylcholinesterase. These drugs are used for their effects on skeletal muscle
and the eye (pupillary constriction and decreased intraocular pressure) and for the
treatment of atropine poisoning. On the other hand, diisopropyl fluorophosphate,
which has military potential as a powerful nerve gas poison, inactivates
acetylcholinesterase for weeks, which makes this a particularly lethal poison.
Generally the pharmacological properties of
anticholinesterase agents can be predicted merely by knowing those loci where
acetylcholine is released physiologically by nerve impulses. and the responses of the
corresponding effector organs to acetylcholine. While this is true of the main, the
diverse locations of cholinergic synapses increase the complexity of the response.
Potentially, the antcholiesterase agents can produce all of the following effects:
1) stimulation of muscarinic receptor responses at autonomic effector organs;
2) stimulation, followed by paralysis, of all autonomic ganglia and skeletal muscle;
and 3) stimulation, with occasional subsequent depression, of cholinergic receptor
sites in the CNS. However, with smaller doses, particularly
those employed therapeutically, several modifying factors are significant.
Neostigmine and Pydrostigmine
Neostigmine and pydrostigmine are among the principal
anticholinesterases. These drugs have only a few clinical uses, mainly in augmenting
gastric and intestinal contractions (in treatment of obstructions of the digestive tract),
in generally augmenting muscular contractions (in the treatment
of myasthenia gravis), and in constricting the eye pupils (in the treatment of
glaucoma). Other anticholinesterases in larger doses, however, are widely used as toxins
that achieve their effects by causing a continual stimulation of the parasympathetic
nervous system. Parathion and malathion are thus highly effective agricultural
insecticides, while tabun and serin are nerve gases used in chemical warfare to induce
nausea, vomiting, convulsions, and death in humans. This action produces a decrease
in the rate of destruction of acetylcholine in the synaptic cleft and hence an increase in
the amount of transmitter available to interact with the receptors.
Serin Nerve Gas
 |
Mechanism of nerve gases
(serin) on Acetylcholinesterase |
 |
General Information
(follow this guy's links) |
E.
Acetylcholine Antagonists
Atropine
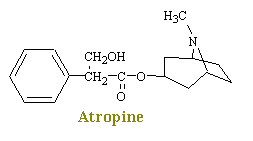
Atropine, a naturally occurring alkaloid of "Atropa belladonna", the
deadly nightshade, inhibits the actions of acetylcholine on autonomic effectors
innervated by postganglionic cholinergic nerves as well as on smooth muscles that lack
cholinergic innervation. Since atropine antagonizes the muscarinic actions of
acetylcholine, it is known as an antimuscarinic agent. Evidence shows that atropine and related compounds compete with
muscarinic agonists for identical binding sites on muscarinic receptors.
In general, antimuscarinic agents have only a moderate effect on the actions of
acetylcholine at nicotinic receptor sites. Thus, at autonomic ganglia, where
transmission primarily involves an action of acetylcholine on nicotinic receptors,
atropine produces only partial block. At the neuomuscular junction, where the
receptors are exclusively nicotinic, extremely high doses of atropine or related drugs are
required to cause any degree of blockade. In the central nervous system, cholinergic
transmission appears to be predominantly nicotinic in the spinal chord and both nicotinic
and muscarinic in the brain. Many of the CNS effects of atropine-like drugs are
probably attributable to their central antimuscarinic actions.
When used as premedication for anaesthesia, atropine
decreases bronchial and salivary secretions, blocks the bradycardia associated with some
drugs used in anaesthesia such as halothane, suxamethonium and neostigmine, and also helps
prevent bradycardia from excessive vagal stimulation.
There is usually an increase in heart rate and sometimes a
tachycardia as well as inhibition of secretions (causing a dry mouth) and relaxation of
smooth muscle in the gut, urinary tract and biliary tree. Since atropine crosses the blood
brain barrier CNS effects in the elderly may include amnesia, confusion and excitation.
Pupillary dilatation and paralysis of accommodation occur, with an increase in
intraocular pressure especially in patients with glaucoma. Occasionally small intravenous
doses may be accompanied by slowing of the heart rate due to a central effect - this
resolves with an extra increment of intravenous atropine Being a sympathetic
cholinergic blocking agent, signs of parasympathetic block may occur such as dryness of
the mouth, blurred vision, increased intraocular tension and urinary retention.
Atropine Nerve Gas Treatment
Sarin is a nerve agent in the organophosphate
family. It is dispersed in a droplet or mist form. Upon inhalation, for instance,
the symptoms (in order of occurance) include: runny nose, bronchial secretions, tightness
in the chest, dimming of vision, pin-point pupils, drooling, excessive perspiration,
nausea, vomiting, involuntary defecationand urination, muscle tremors, convulsions,
coma, and death. Primary treatment for nerve agents is atropine sulfate. It is
commonly carried in auto-injectors (see picture) by military personnel in dosages of 1-2
mgs. However, in many cases, massive doses may be necessary to reverse the effects
of the anticholinesterase agents. Frequently, 20-40 mgs. of atropine may be
necessary.
 |
| Picture of
Auto-Injector |
Curare
In 1799 the famous Prussian explorer and scientist Baron
Von Humboldt discovered a potent drug called curare. On an expedition into the jungles of
Venezuela, he watched an Indian hunter bring down a large animal with a single shot from
his bow and arrow. The arrow had been poisoned with curare, a potion with two curious
properties, derived from the jungle plants. Curare injected into the bloodstream, as it
was when hunting animals, was deadly. It immobilized the body, attacked the vital organs,
and caused death almost instantaneously. Humboldt discovered the second property of curare
in a more dramatic fashion. He became sick, and a native witch doctor forced Humboldt to
drink some curare that had been diluted with water. Terrified that he was going to die,
Humboldt was surprised to find that after drinking the curare, he felt significantly
better. Curare, when it was diluted and taken orally, he discovered, could have a positive
medicinal value without causing any damage to vital organs.
Curare is a generic term for various South American arrow
poisons. The main active ingredient in curare is d-tubocurarine, which has the chemical
structure shown below.
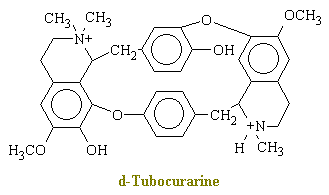
In brief, d-tubocurarine is an antagonist of the
cholinergic receptor sites at the post junctional membrane and thereby blocks
competitively the transmitter action of acetylcholine. When the drug is applied
directly to the end-plate of a single isolated muscle fiber under microscopic control, the
muscle cell becomes insensitive to motor-nerve impulses and to direct applied
acetylcholine; however, the end -plate region and the remainder of the muscle fiber
membrane retain their normal sensitivity to the application of potassium ions, and the
muscle fiber still responds to direct electrical stimulation. Because acetylcholine
release into the neuromuscular junction muscle is what initiates contraction, curare
causes muscle relaxation and paralysis.
There are several clinical application for
neuromuscular blockage. The most important by far is the induction of muscle relaxation
during anesthesia for effective surgery. Without such drugs deeper anesthesia,
requiring more anesthetic, would be needed to achieve the same degree of muscle
relaxation: tracheal intubation would also be impossible because of strong reflex response
to tube insertion. It is the decreased need for anesthetics, however, that
represents increased surgical safety.
Neuromuscular blockers also find limited
utility in convulsive situations such as those precipitated by tetanus infections and to
minimize injury to patients undergoing electroconvulsive therapy. Manipulation of
fractured or dislocated bones may also be aided by such drugs.
Botulus Toxin
Botulus toxin* is a bacterial poison that
prevents the release of acetylcholine by all types of cholinergic nerve terminals.
Apparently, the toxin blocks release of vesicular acetylcholine at the preterminal portion
of the axon, but why this is confined to cholinergic fibers is not known.
Miscellaneous
Site With a Whole Bunch of Stuff on Various Cholinergic Drugs, and
More!!!





